How to Use Electrodes: Examples, Pinouts, and Specs

 Design with Electrodes in Cirkit Designer
Design with Electrodes in Cirkit DesignerIntroduction
Electrodes are conductive materials designed to facilitate the flow of electric current into or out of a medium. They serve as an interface between an electrical circuit and the medium, which can be a solid, liquid, or gas. Electrodes are essential in a wide range of applications, including energy storage, chemical reactions, and sensing technologies.
Explore Projects Built with Electrodes
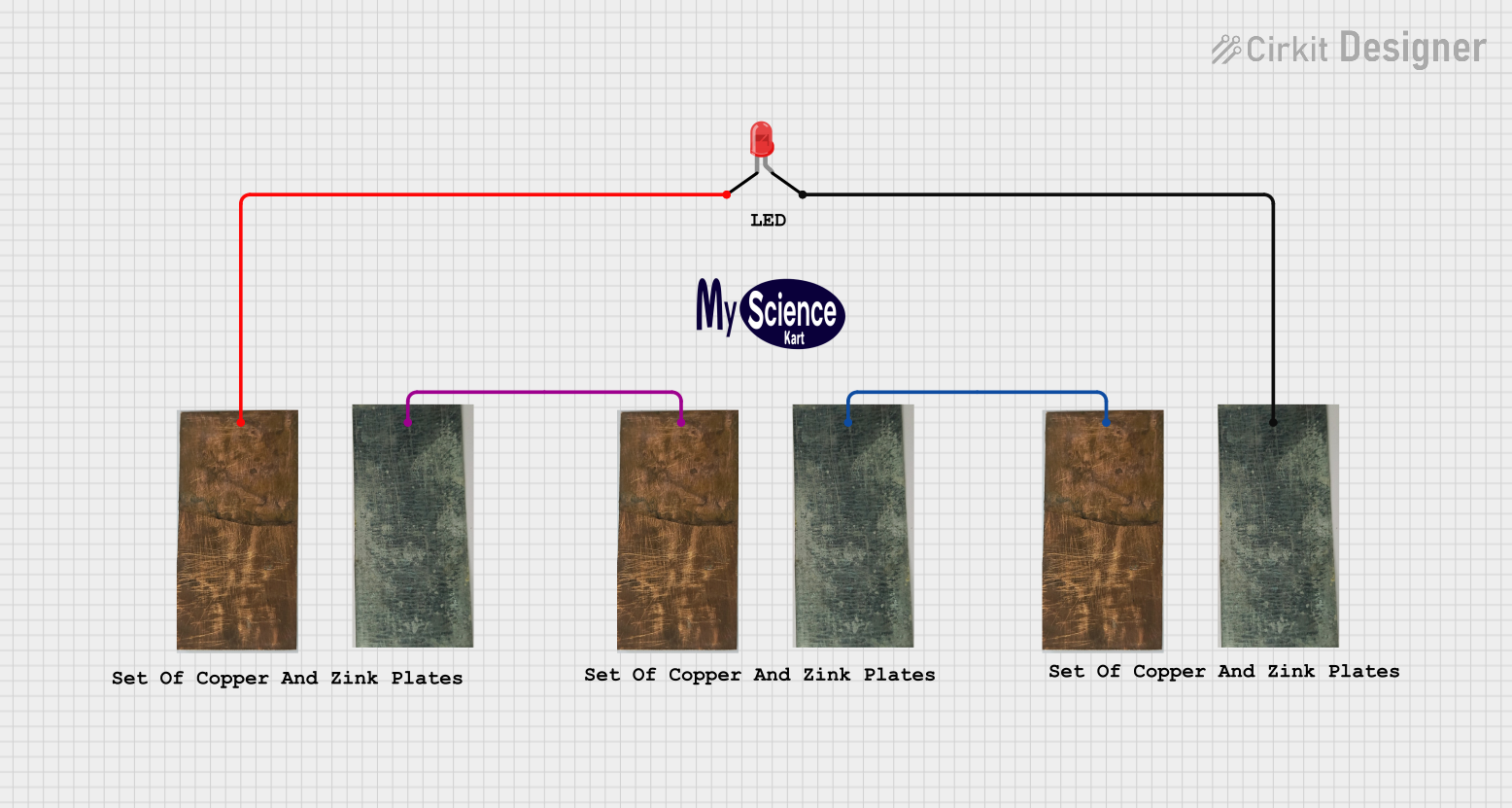
 Open Project in Cirkit Designer
Open Project in Cirkit Designer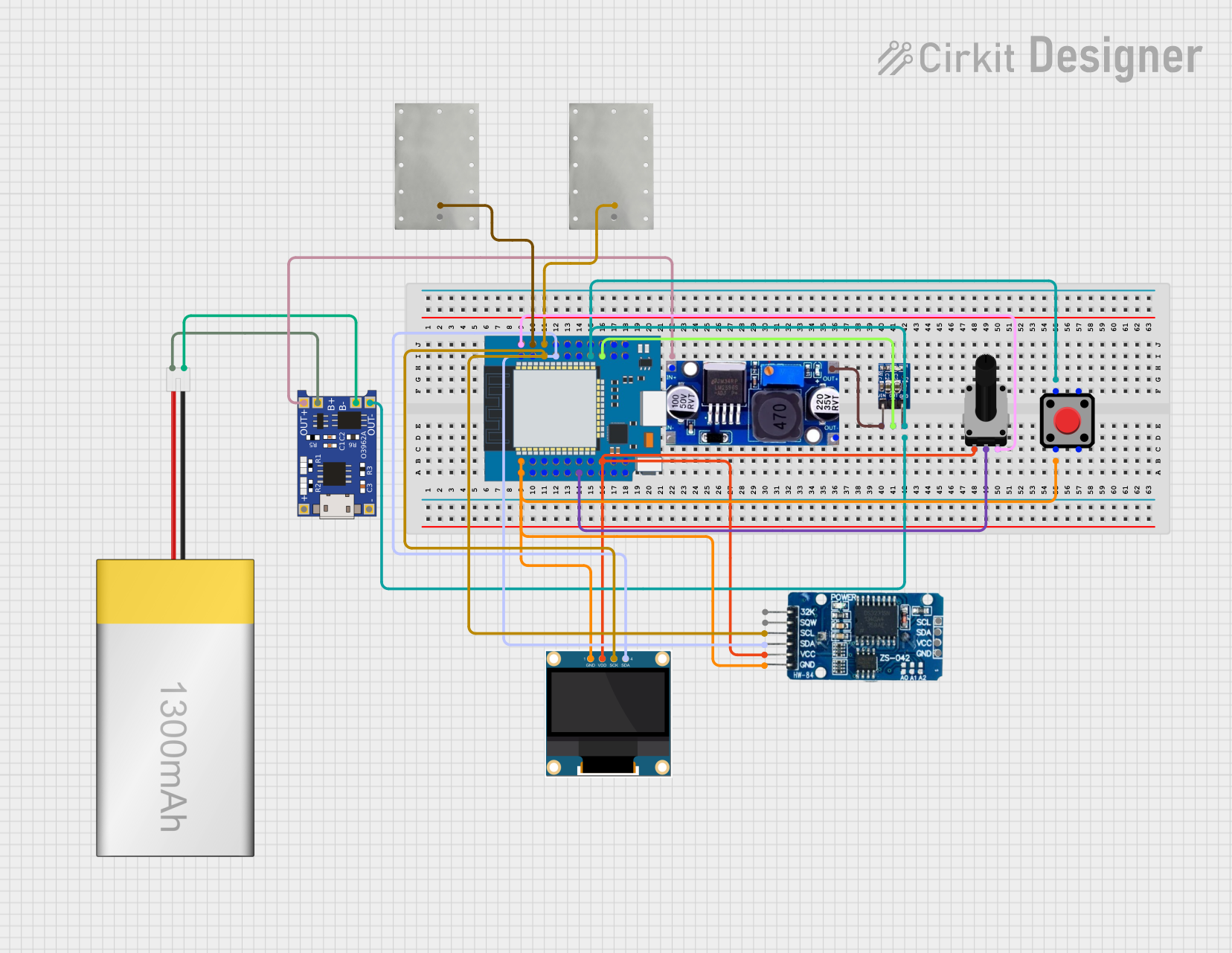
 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit Designer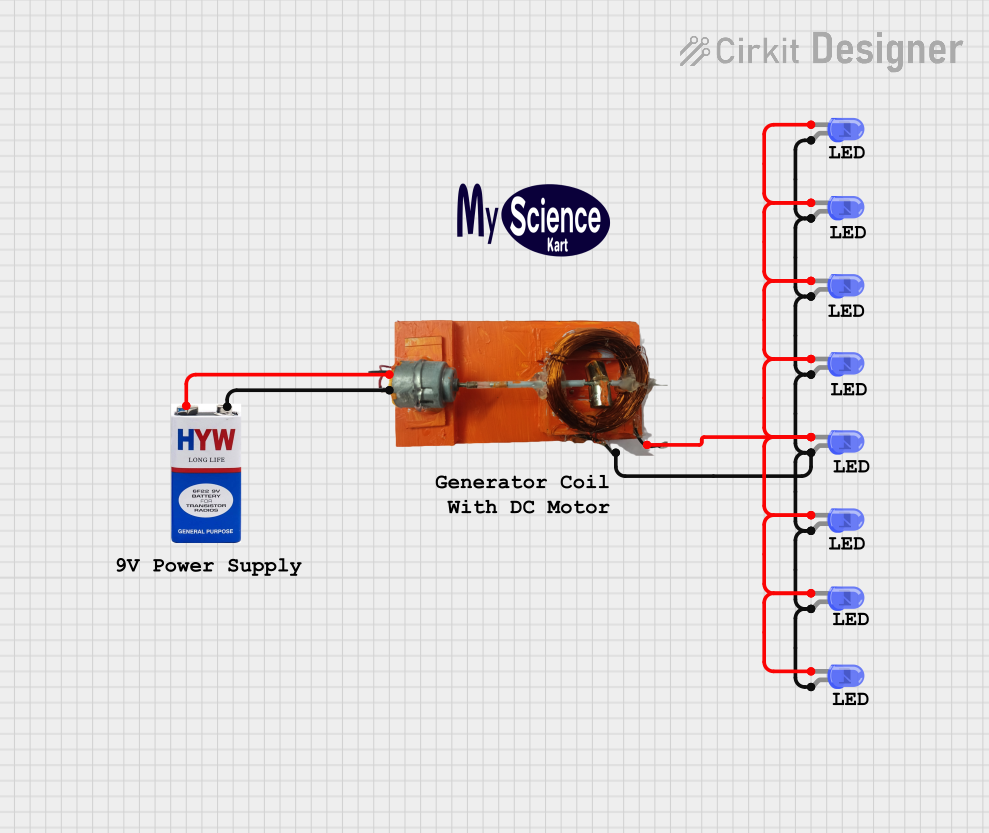
 Open Project in Cirkit Designer
Open Project in Cirkit DesignerExplore Projects Built with Electrodes

 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit DesignerCommon Applications and Use Cases
- Batteries: Electrodes act as the anode and cathode, enabling the flow of electrons during charge and discharge cycles.
- Electrolysis: Used to drive chemical reactions by passing an electric current through a solution.
- Sensors: Electrodes are integral in devices like pH meters, ECG machines, and gas sensors.
- Capacitors: Serve as plates to store electrical energy.
- Welding: Used to conduct current and create heat for joining materials.
Technical Specifications
The specifications of electrodes vary depending on their material, size, and intended application. Below are general technical details:
Key Technical Details
- Material: Common materials include graphite, platinum, gold, silver, copper, and carbon.
- Conductivity: High electrical conductivity is essential for efficient current flow.
- Voltage Range: Typically depends on the application (e.g., 1.5V–12V for batteries, higher for industrial processes).
- Current Capacity: Varies based on size and material, ranging from milliamps to hundreds of amps.
- Durability: Resistance to corrosion and wear is critical, especially in chemical environments.
Pin Configuration and Descriptions
Electrodes do not have a standard pin configuration like ICs or transistors. However, they are typically connected to circuits via terminals or leads. Below is an example of a basic electrode setup for a two-electrode system:
| Electrode | Description |
|---|---|
| Anode | The positive electrode where oxidation occurs (loss of electrons). |
| Cathode | The negative electrode where reduction occurs (gain of electrons). |
| Lead/Terminal | Conductive wire or connector used to interface the electrode with the circuit. |
Usage Instructions
How to Use Electrodes in a Circuit
- Identify the Type of Electrode: Determine whether the electrode will act as an anode or cathode based on the application.
- Connect to the Circuit:
- Use conductive wires or terminals to connect the electrodes to the power source or sensing device.
- Ensure proper polarity (positive to anode, negative to cathode) to avoid reverse operation.
- Immerse in Medium:
- For applications like electrolysis or sensing, immerse the electrodes in the appropriate medium (e.g., electrolyte solution).
- Ensure the electrodes are clean and free of contaminants for optimal performance.
- Apply Voltage/Current:
- Use a power source to apply the required voltage or current to the electrodes.
- Monitor the system to ensure safe operation within the specified limits.
Important Considerations and Best Practices
- Material Selection: Choose electrode materials compatible with the medium to prevent corrosion or degradation.
- Surface Area: Larger surface areas improve efficiency in applications like electrolysis and sensing.
- Cleaning: Regularly clean electrodes to remove deposits or contaminants that may affect performance.
- Safety: Handle electrodes with care, especially in high-voltage or chemical environments.
Example: Using Electrodes with an Arduino UNO
Electrodes can be used with an Arduino UNO for sensing applications, such as measuring the conductivity of a solution. Below is an example code snippet:
// Example: Measuring solution conductivity using electrodes and Arduino UNO
// Connect one electrode to pin A0 and the other to GND
const int electrodePin = A0; // Analog pin connected to the electrode
int sensorValue = 0; // Variable to store the analog reading
void setup() {
Serial.begin(9600); // Initialize serial communication at 9600 baud
}
void loop() {
// Read the analog value from the electrode
sensorValue = analogRead(electrodePin);
// Convert the analog value to a voltage (assuming 5V reference)
float voltage = sensorValue * (5.0 / 1023.0);
// Print the voltage to the Serial Monitor
Serial.print("Electrode Voltage: ");
Serial.print(voltage);
Serial.println(" V");
delay(1000); // Wait for 1 second before the next reading
}
Troubleshooting and FAQs
Common Issues
Poor Conductivity:
- Cause: Dirty or corroded electrodes.
- Solution: Clean the electrodes with a suitable cleaning agent (e.g., isopropyl alcohol or distilled water).
Incorrect Readings:
- Cause: Improper connection or damaged electrodes.
- Solution: Verify connections and inspect electrodes for physical damage.
Electrode Degradation:
- Cause: Use of incompatible materials in a corrosive medium.
- Solution: Select electrodes made of corrosion-resistant materials like platinum or graphite.
Overheating:
- Cause: Excessive current or voltage.
- Solution: Operate within the specified current and voltage limits.
FAQs
Q: Can I use any material as an electrode?
A: No, the material must have high conductivity and be compatible with the medium to avoid corrosion or degradation.Q: How do I clean electrodes?
A: Use a soft cloth and a cleaning agent like isopropyl alcohol or distilled water. Avoid abrasive materials that may damage the surface.Q: What is the difference between an anode and a cathode?
A: The anode is the positive electrode where oxidation occurs, while the cathode is the negative electrode where reduction occurs.Q: Can electrodes be reused?
A: Yes, electrodes can be reused if they are properly cleaned and maintained.Q: How do I know if an electrode is damaged?
A: Signs of damage include visible corrosion, cracks, or inconsistent performance in the circuit. Replace damaged electrodes promptly.