How to Use Electrode: Examples, Pinouts, and Specs

 Design with Electrode in Cirkit Designer
Design with Electrode in Cirkit DesignerIntroduction
An electrode is a conductor through which electricity enters or leaves an electrochemical cell or other device, facilitating the flow of current. Electrodes are essential components in a wide range of applications, including batteries, fuel cells, electroplating, and medical devices such as ECG and EEG sensors. They serve as the interface between the electronic circuit and the medium (e.g., electrolyte or biological tissue) where electrochemical reactions occur.
Common applications and use cases:
- Batteries (e.g., lithium-ion, lead-acid)
- Electroplating and electrolysis
- Fuel cells and supercapacitors
- Medical devices (e.g., ECG, EEG, and TENS units)
- Sensors for chemical and biological analysis
Explore Projects Built with Electrode
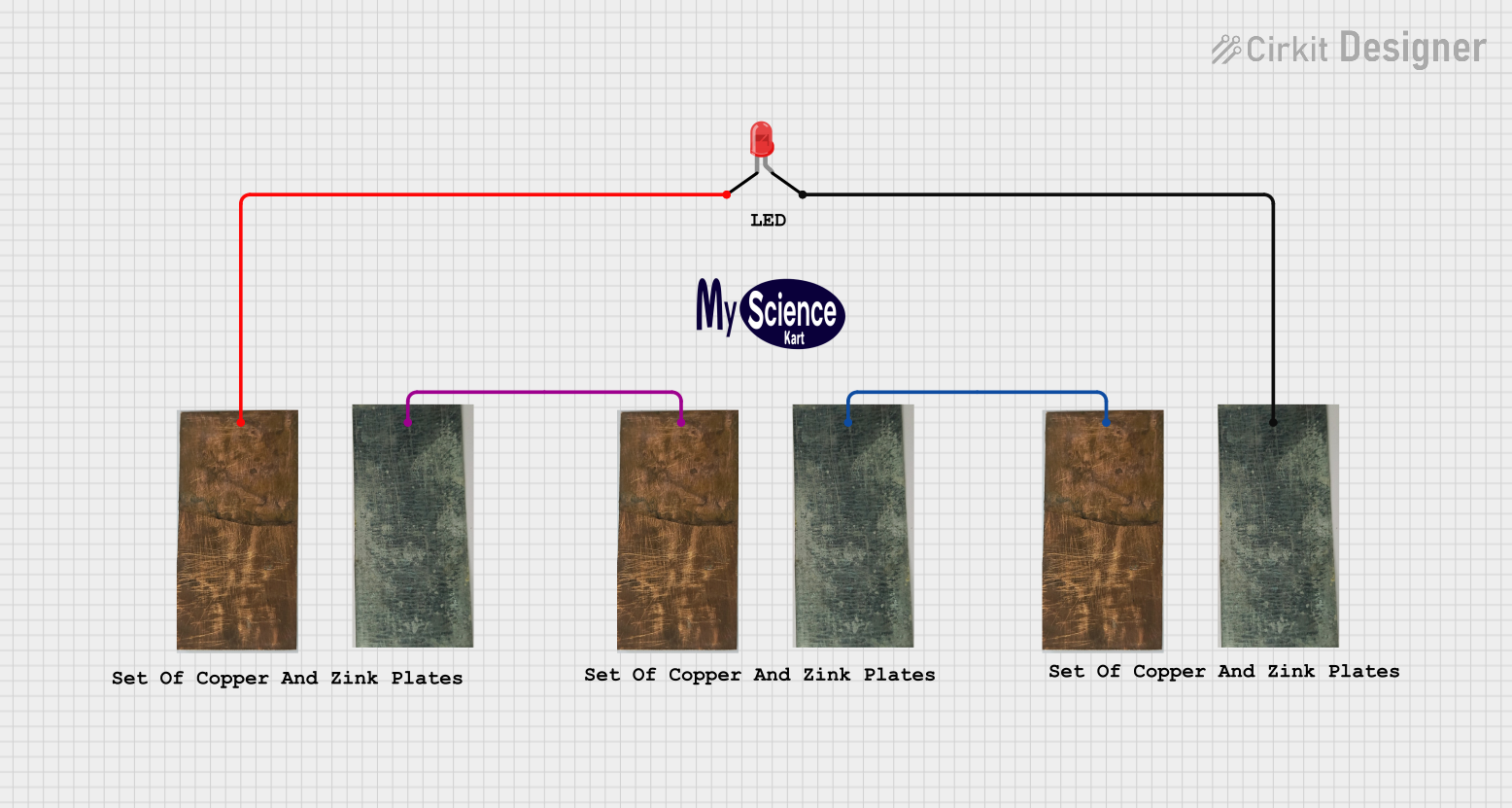
 Open Project in Cirkit Designer
Open Project in Cirkit Designer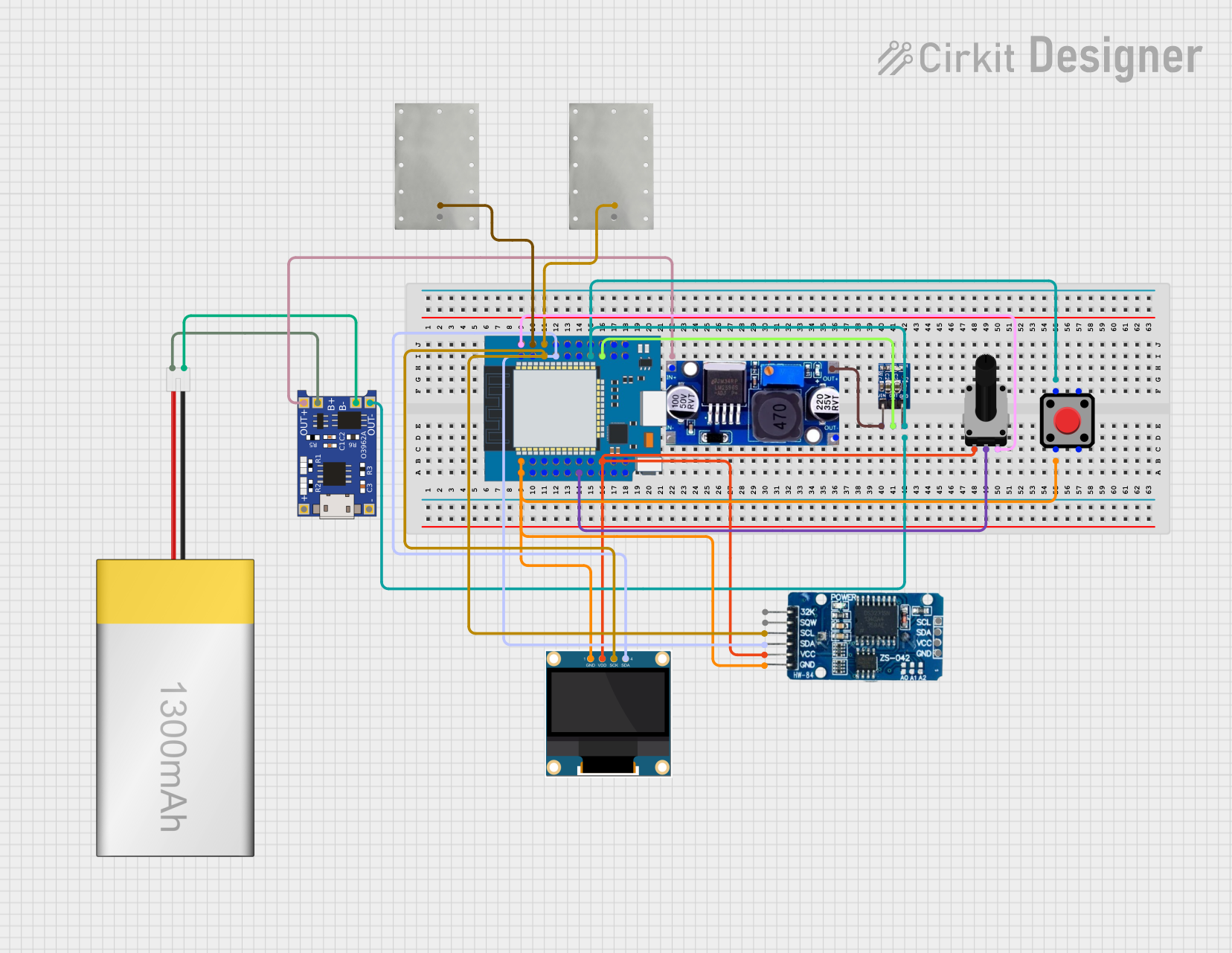
 Open Project in Cirkit Designer
Open Project in Cirkit Designer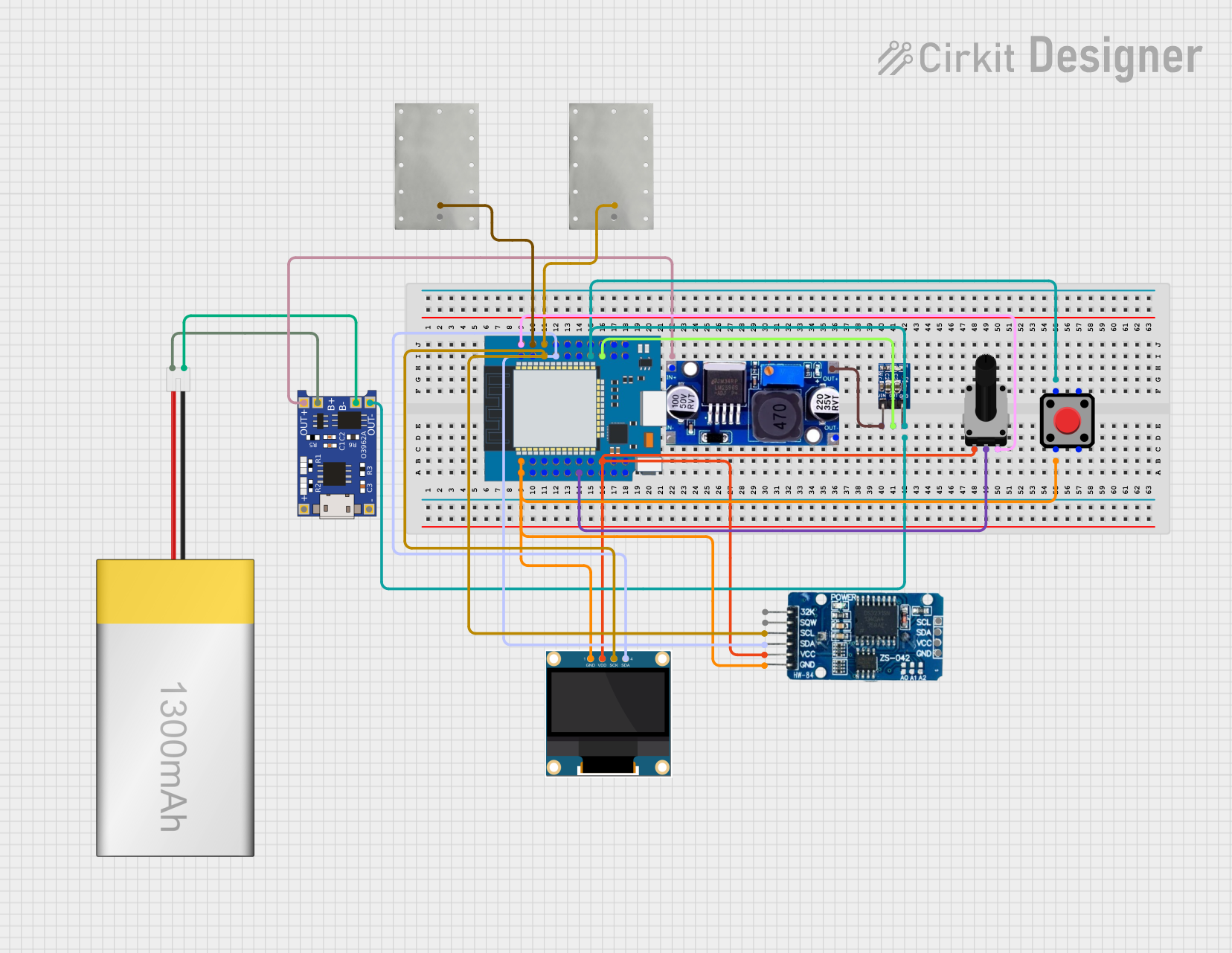
 Open Project in Cirkit Designer
Open Project in Cirkit Designer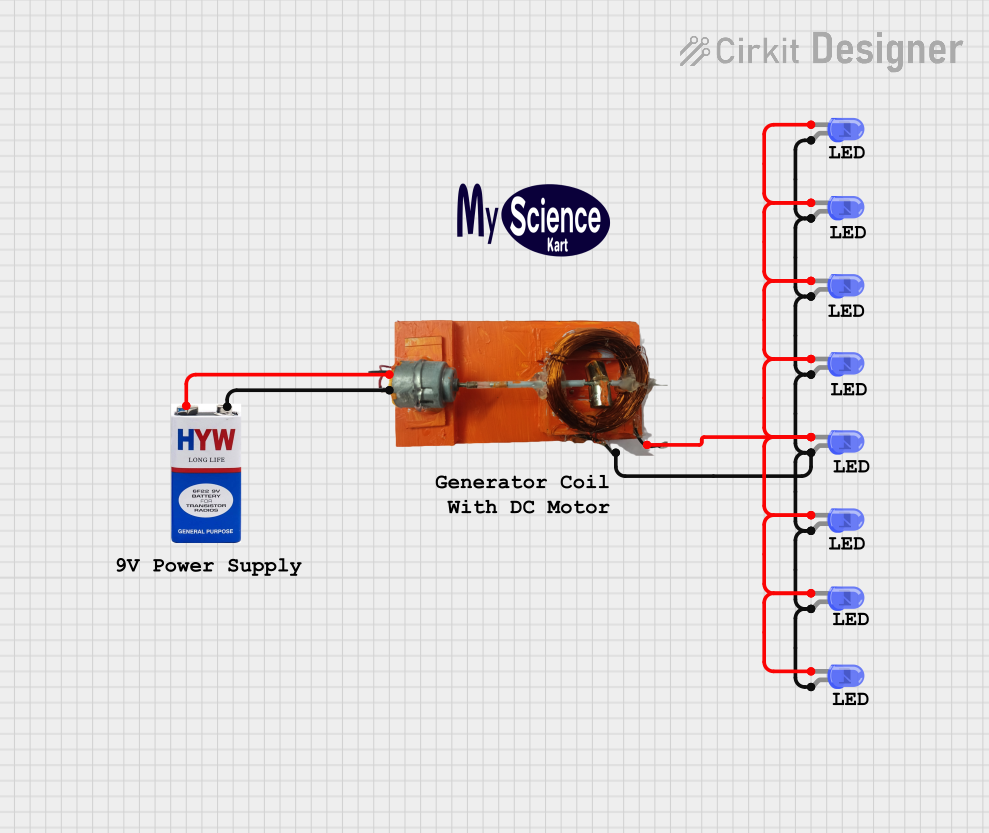
 Open Project in Cirkit Designer
Open Project in Cirkit DesignerExplore Projects Built with Electrode

 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit Designer
 Open Project in Cirkit Designer
Open Project in Cirkit DesignerTechnical Specifications
The technical specifications of an electrode depend on its material, size, and intended application. Below are general specifications for common electrode types:
| Parameter | Description |
|---|---|
| Material | Graphite, platinum, gold, silver, copper, or other conductive materials |
| Conductivity | High electrical conductivity (varies by material) |
| Operating Voltage Range | Typically 0–5V for sensors; higher for industrial applications |
| Current Capacity | Depends on size and material; ranges from microamps (sensors) to amps (batteries) |
| Resistance | Low resistance for efficient current flow |
| Durability | Corrosion resistance depends on material (e.g., platinum is highly durable) |
Pin Configuration and Descriptions
Electrodes typically do not have a standard pin configuration, as their design varies by application. However, in circuits, electrodes are often connected via leads or terminals. Below is an example of a two-electrode system:
| Pin/Terminal | Description |
|---|---|
| Positive (+) | The anode, where oxidation occurs (electrons leave the electrode) |
| Negative (-) | The cathode, where reduction occurs (electrons enter the electrode) |
For three-electrode systems (e.g., in electrochemical analysis), the configuration includes:
| Pin/Terminal | Description |
|---|---|
| Working Electrode | The electrode where the reaction of interest occurs |
| Counter Electrode | Completes the circuit by allowing current to flow |
| Reference Electrode | Provides a stable reference potential for accurate measurements |
Usage Instructions
How to Use the Electrode in a Circuit
- Identify the type of electrode: Determine whether you are using a two-electrode or three-electrode system.
- Connect the electrodes:
- For a two-electrode system, connect the positive terminal to the anode and the negative terminal to the cathode.
- For a three-electrode system, connect the working, counter, and reference electrodes to the appropriate terminals of your measurement device.
- Ensure proper contact: Use conductive clips, wires, or connectors to ensure a secure and low-resistance connection.
- Apply voltage or current: Use a power supply or measurement device to apply the desired voltage or current to the electrodes.
- Monitor the system: Observe the electrochemical reaction or signal output, depending on the application.
Important Considerations and Best Practices
- Material selection: Choose an electrode material compatible with the medium and application (e.g., platinum for corrosive environments).
- Surface preparation: Clean the electrode surface to remove contaminants and ensure accurate results.
- Avoid overloading: Do not exceed the electrode's current or voltage rating to prevent damage.
- Polarity: Ensure correct polarity when connecting electrodes to avoid unintended reactions.
- Storage: Store electrodes in a clean, dry environment to prevent corrosion or degradation.
Example: Using an Electrode with Arduino UNO
Below is an example of using a simple electrode sensor (e.g., pH sensor) with an Arduino UNO:
// Example code for reading an analog signal from an electrode sensor
// connected to an Arduino UNO. The sensor is connected to analog pin A0.
const int electrodePin = A0; // Define the analog pin for the electrode
int sensorValue = 0; // Variable to store the sensor reading
void setup() {
Serial.begin(9600); // Initialize serial communication at 9600 baud
}
void loop() {
sensorValue = analogRead(electrodePin); // Read the analog value from the electrode
float voltage = sensorValue * (5.0 / 1023.0); // Convert the reading to voltage
// Print the sensor value and voltage to the Serial Monitor
Serial.print("Sensor Value: ");
Serial.print(sensorValue);
Serial.print(" | Voltage: ");
Serial.println(voltage);
delay(1000); // Wait for 1 second before the next reading
}
Troubleshooting and FAQs
Common Issues and Solutions
No signal or incorrect readings:
- Check the connections to ensure the electrodes are securely attached.
- Verify that the electrode surface is clean and free of contaminants.
- Ensure the power supply or measurement device is functioning correctly.
Corrosion or degradation of the electrode:
- Use electrodes made of corrosion-resistant materials for harsh environments.
- Store electrodes properly when not in use.
Unstable or noisy signal:
- Use shielded cables to reduce electrical noise.
- Ensure the reference electrode (if applicable) is functioning properly.
Incorrect polarity:
- Double-check the wiring to ensure the correct polarity is applied.
FAQs
Q: Can I use any material as an electrode?
A: No, the material must be conductive and compatible with the medium and application. For example, graphite and platinum are commonly used for their stability and conductivity.
Q: How do I clean an electrode?
A: Use a soft cloth or brush with distilled water or a suitable cleaning solution. Avoid abrasive materials that could damage the electrode surface.
Q: What is the difference between a working electrode and a counter electrode?
A: The working electrode is where the reaction of interest occurs, while the counter electrode completes the circuit and allows current to flow.
Q: Can electrodes be reused?
A: Yes, electrodes can often be reused if they are properly cleaned and maintained. However, some applications may require disposable electrodes for accuracy or hygiene.